Post-Market Surveillance of Medical Devices
Search, analyze, and export relevant events from FDA Recalls, MAUDE, BfArM, MHRA, Pubmed, Europe PMC, ClinicaTrials.gov, and more data sources. All Recall, Adverse Events, Cybersecurity, and Scientific Literature databases you need in one place.
Exciting News for PMM
ACQUISITION ANNOUNCEMENT
Our curated data store and advanced analytics technology have been purchased by a private party. As part of this transition, the public application has been retired. We are incredibly proud of the technology we built and deeply grateful to our community of users who supported us on this journey.

.png)
.png)



.png)

.png)
.png)



.png)
Get results with half the effort
Access a unified platform connecting top data sources like FDA Recalls, MAUDE, BfArM, MHRA, PubMed, Europe PMC, and ClinicalTrials.gov—all in one place. Need more? Contact us to add additional databases.
Find the most comprehensive insights on medical device events, research articles, and cybersecurity vulnerabilities to enhance product development and post-market surveillance.
Go beyond public BfArM and MHRA databases with advanced OCR, extracting data from scanned PDFs to uncover hidden, non-searchable information. Don’t miss critical insights.

Unlock deeper insights and streamline your post-market surveillance with our robust advanced search capabilities.
Easily define specific search parameters across companies, products, FDA product codes, keywords, device problems, and more.
Break through 10x the FDA's 500-record limit to access comprehensive data and uncover critical insights.
Our intuitive search tool empowers you to quickly locate relevant safety events, potential vulnerabilities, and supporting scientific literature.
.png)
Unlock powerful insights with robust analytics, interactive trend charts, and comparison tools to quickly analyze and uncover key events.
Our intuitive filtering options let you sift through thousands of records using product name, manufacturer name, keywords, or even custom tags.
Leverage advanced filter capabilities for precise queries to locate specific events or explore broader topics to streamline your data analysis.
Identify Use Errors instantly with our AI-powered detection tools, ensuring proactive risk management.
Stay ahead with smart Alerts and Notifications—get daily updates on new adverse events, recalls, scientific publications, or cybersecurity vulnerabilities that align with your saved searches.

Easily export comprehensive data for customized reporting, offline analysis, or seamless integration with your quality management systems. Download presentation-ready charts.
Ensure compliance with our comprehensive audit trail, automatically tracking every entry and decision for full transparency and reproducibility in your post-market reviews.
Achieve superior data integrity for regulatory submissions.

KNOWN USE ERROR
.png)
AI-Powered Screening
Identify Use Errors instantly with AI-powered detection tools. Our system automatically highlights possible Use Errors, pinpoints specific use error keywords in the event data, and provides clear explanations of the use error to help you understand and address risks proactively.
.png)
Critical User Actions
Uncover root causes with AI-powered analysis of Use Errors. Our algorithm identifies user actions leading to errors, the resulting failures, and generates a correlation map. Quickly gain deep insights into the most common actions and failures to drive informed improvements.
.png)
Import Internal Complaint Data
Enhance your analysis by importing internal customer complaint data into our platform. Easily import, format, and validate your data, then leverage our AI-powered Use Error analysis tools to detect use errors, user actions, failures, and correlations—unlocking actionable insights for proactive risk management.
The Best Solution for Post-Market Surveillance of Medical Devices
Centralized Post-Market Surveillance Analysis
Access all relevant data—from complaints to literature reviews—in one powerful platform, streamlining your workflow and saving you valuable time.
Customizable Data Searches
Define precise search criteria across companies, product categories, and keywords. Save searches, analyze results, monitor trends, and find relevant events.
Real-Time Updates for Proactive Risk Mitigation
Track product performance with real-time updates and alerts, enabling you to proactively identify and address potential safety issues and maintain regulatory compliance.
Actionable Insights with Advanced Analytics
Make informed decisions with robust analytics, interactive trend charts, and comparison tools, empowering you to proactively improve product quality and patient safety.
Flexible Data Export for Deeper Analysis
Export comprehensive data sets in multiple formats for customized reporting, offline analysis, and seamless integration with your existing quality management systems.
Enhanced Product Quality & Patient Safety
Proactively identify and resolve product quality issues, leading to improved device reliability, enhanced patient safety, and demonstrable regulatory compliance.
Medical Device Insights
Expert Insights & Resources for Medical Device Safety
Try the Professional Plan Free for 2 Weeks!
Explore all features of PMM for 2 weeks to see how it can simplify your post-market surveillance. If you cancel before the trial ends, your credit card will not be charged



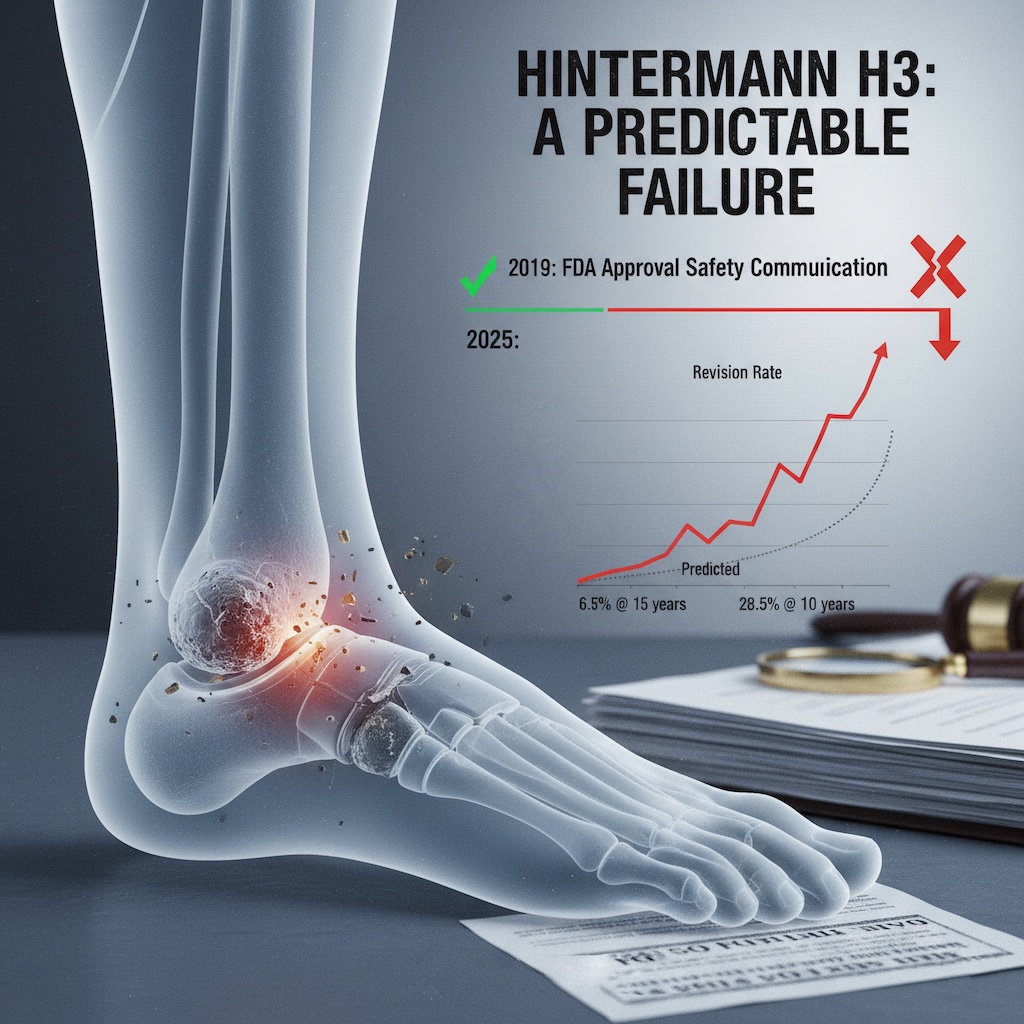

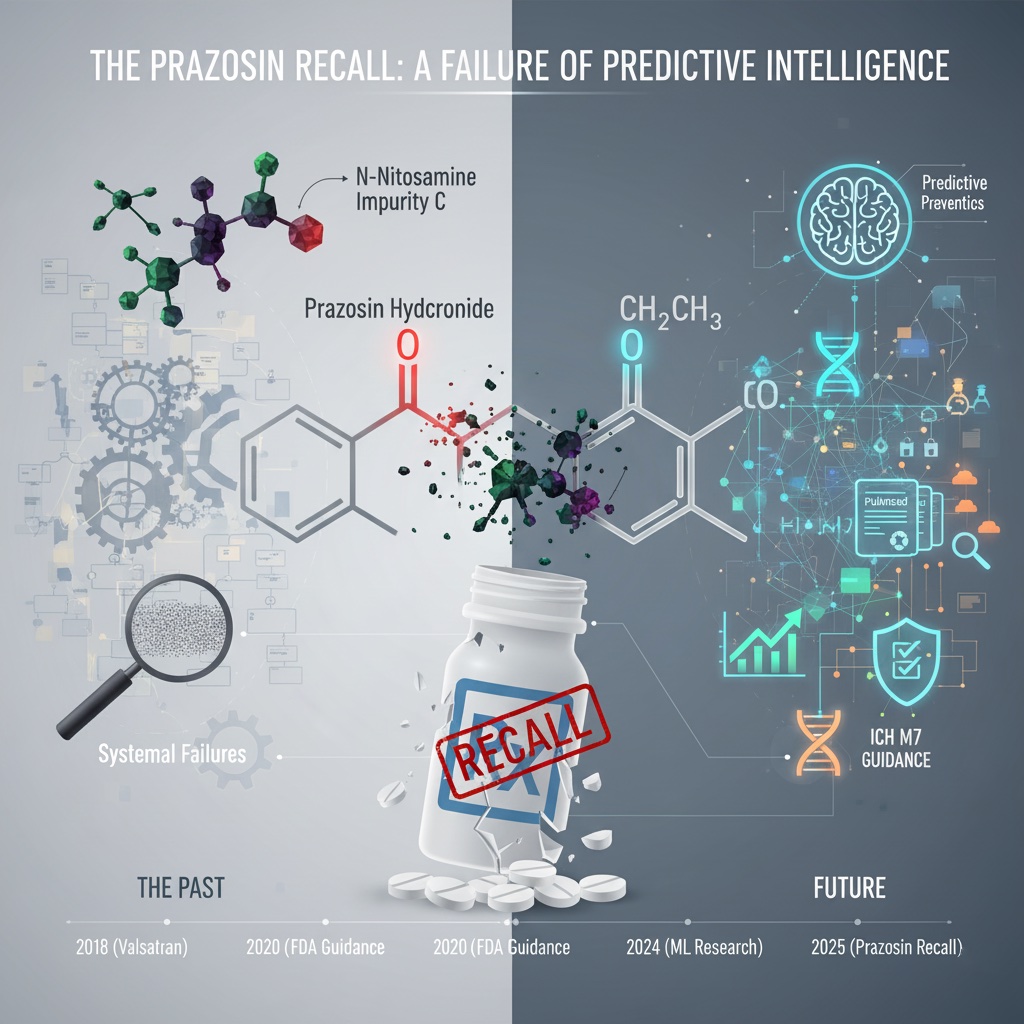


.png)